Blog
How to Prepare for a Successful FDA Pre-Submission Meeting
An FDA Pre-Submission (Pre-Sub) meeting is a critical opportunity for medical startups to receive early feedback before submitting a regulatory application. Proper preparation—including a strong submission package, clear objectives, and well-structured questions—can streamline the approval process, reduce delays, and improve your chances of success. This guide walks you through the essential steps to ensure a productive Pre-Sub meeting.
Understanding FDA 510(k) vs. De Novo: Which Pathway is Right for Your Medical Device?
Navigating FDA approval for a medical device means choosing the right regulatory pathway. The 510(k) process is ideal for moderate-risk devices with a market equivalent, while the De Novo pathway suits innovative devices without a predicate. Understanding the differences in cost, approval time, and requirements can streamline your strategy and speed up market entry.
Regulatory Basics for Medical Startups: A Simplified Guide
Regulatory compliance is a critical step for medical startups entering the market. Understanding device classification, FDA submission pathways, and quality management requirements can help streamline the approval process and prevent costly delays. This guide outlines the essential regulatory basics to help startups navigate compliance effectively.
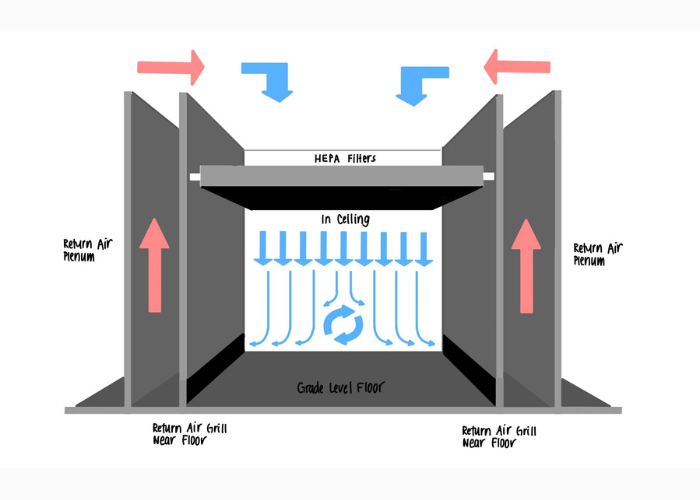
Must-Have Equipment for Cleanroom Facilities
Equipping a cleanroom with the right tools is essential for maintaining contamination control and operational efficiency. From HEPA filters and airflow systems to sterilization equipment and specialized furniture, these items are crucial for ensuring compliance with industry standards. This blog explores the top must-have equipment every cleanroom facility needs to operate effectively.
ISO Standards Demystified: Choosing the Right Cleanroom Classification
ISO cleanroom standards, such as ISO 14644-1, categorize cleanrooms by air cleanliness levels, ensuring they meet industry-specific requirements. From ultra-clean environments for semiconductor manufacturing to controlled spaces for general assembly, understanding these classifications is essential for compliance and efficiency. This blog simplifies the classifications, helping you select the right one for your needs.
Breaking Down Contamination Risks in Cleanrooms
Cleanrooms are vital for contamination-sensitive industries, but understanding and addressing contamination risks is essential to maintain operational integrity. From particulate and microbial contaminants to chemical residues, each poses unique challenges. This blog explores the sources, types, and prevention strategies to help minimize contamination risks and ensure cleanroom efficiency.
The Importance of Environmental Monitoring in Regulatory Compliance
Environmental monitoring (EM) is crucial for ensuring regulatory compliance in industries like pharmaceuticals, biotechnology, and medical devices. By tracking airborne particles, microbial contamination, and environmental factors such as temperature and pressure, EM safeguards product quality, protects patient safety, and meets stringent standards like ISO 14644 and FDA GMP guidelines. Learn how robust monitoring practices and advanced technologies can help maintain controlled environments and regulatory adherence.
The Future of Cleanroom Technology: Trends and Insights
The cleanroom technology market is set to grow from $4.1 billion in 2023 to $7.2 billion by 2033, driven by advancements in modular designs, AI monitoring systems, and energy-efficient solutions. Key regions like North America lead the market, while rapid adoption in India and China highlights global expansion. Despite high setup costs, opportunities in automation and sustainable practices are reshaping the industry.
Cleanroom Maintenance Checklist: Best Practices for Long-Term Efficiency
Maintaining a cleanroom is essential for ensuring long-term efficiency and compliance with industry standards. From daily surface cleaning to annual comprehensive validations, following a structured maintenance checklist can prevent contamination, extend equipment life, and protect sensitive processes. This guide outlines best practices for cleanroom maintenance, helping you stay ahead of potential issues and ensure consistent performance.
The Science of Airflow: Why It Matters in Cleanroom Design
Airflow plays a critical role in cleanroom design, helping to maintain cleanliness and control contamination. By managing airflow patterns, pressure differentials, and filtration systems, cleanrooms can meet strict industry standards while protecting processes, products, and personnel. This article explores the science behind cleanroom airflow and its importance in achieving controlled environments.
CRISPR Gene Editing: A Breakthrough for Muscular Dystrophy Treatment
A groundbreaking study showcases the potential of CRISPR/Cas9 technology to treat dysferlinopathies, a form of limb-girdle muscular dystrophy. By correcting mutations in the DYSF gene, researchers restored muscle repair functionality, paving the way for personalized genetic therapies.
Optimizing Cell Therapy Manufacturing with the AXP® II System
Discover how the AXP® II System enhances cell therapy manufacturing through automated, closed-system processing. Ideal for cleanroom environments, it ensures precision, efficiency, and compliance in applications like cord blood banking and regenerative medicine.
Understanding ISO Cleanroom Classifications
Navigating ISO cleanroom standards can be challenging, but choosing the right classification is critical for ensuring product quality and regulatory compliance. Learn how to evaluate your industry needs, processes, and costs to select the perfect cleanroom environment.
Biotech Trends Advancing Innovation in Healthcare and Life Science
Biotechnology is evolving rapidly, with advancements like AI-driven drug discovery, CRISPR gene editing, stem cell technology, and personalized medicine redefining healthcare and agriculture. Discover how these trends are transforming the industry and shaping the future of innovation.
Evolution of Cleanroom Technology: Past, Present & Future
Cleanrooms have evolved significantly from basic controlled environments to advanced, tech-driven spaces. Discover how cleanroom technology has progressed over the years, its current applications, and the future innovations shaping industries like pharmaceuticals, biotechnology, and aerospace.
Common Myths About Cleanrooms Debunked
Cleanrooms are vital for industries requiring controlled environments, but misconceptions can lead to inefficiencies and non-compliance. Explore common myths about cleanrooms and learn the facts behind these highly specialized spaces.
CRISPR-Cas9 Explained
CRISPR-Cas9 is transforming genetic research and medicine with its precise DNA-editing capabilities. Acting as a molecular scalpel, this groundbreaking tool offers hope for curing inherited diseases by modifying, deleting, or inserting DNA sequences with unparalleled accuracy.
CRISPR-Cas9: Ownership Disputes and Their Impact on Innovation
CRISPR-Cas9 has revolutionized gene-editing across industries, but unresolved patent disputes complicate its use. Learn how these ownership challenges impact innovation and what strategies businesses can adopt to navigate this complex legal landscape.
Enhancing Laboratory Efficiency: Sorvall™ Legend™ T/RT Centrifuge Buckets
Each ThermoGenesis Cleanroom Suite comes equipped with Sorvall™ Legend™ T/RT Centrifuge Buckets, offering high-capacity, reliable performance for centrifugation needs. These versatile tools support applications across clinical, pharmaceutical, and research industries, ensuring efficiency and precision in controlled environments.
CRISPR Technology Makes Major Advance in Cancer Treatment
Scientists have engineered a breakthrough CRISPR system that can target cancer mutations with single-letter precision. The new technique, developed at the Peter MacCallum Cancer Centre, enables selective destruction of cancer-causing KRAS mutations while preserving healthy genes.
The research tackles a longstanding challenge in cancer treatment: targeting mutations that differ from normal genes by just one letter. Using modified CRISPR-Cas13, researchers achieved 90% reduction in mutated KRAS with minimal impact on normal KRAS. This precision targeting could revolutionize treatment for cancers driven by KRAS mutations, which account for roughly one-third of human cancers.
While the technology shows promise in laboratory settings, researchers are now focusing on developing effective delivery methods to bring this treatment to patients.